THE CLINICAL TRIAL
Bladder cancer is the tenth most common malignancy worldwide. Approximately 25 percent (%) of all new bladder cancer participants present with muscle invasive bladder cancer (MIBC) at the time of diagnosis, and roughly 50% will ultimately develop distant metastases. The SunRISe Clinical Trials was a suite of three clinical studies anti-tumor effects of TAR-200 – an intravesical drug delivery system – in combination with intravenous (IV) cetrelimab and IV cetrelimab alone.
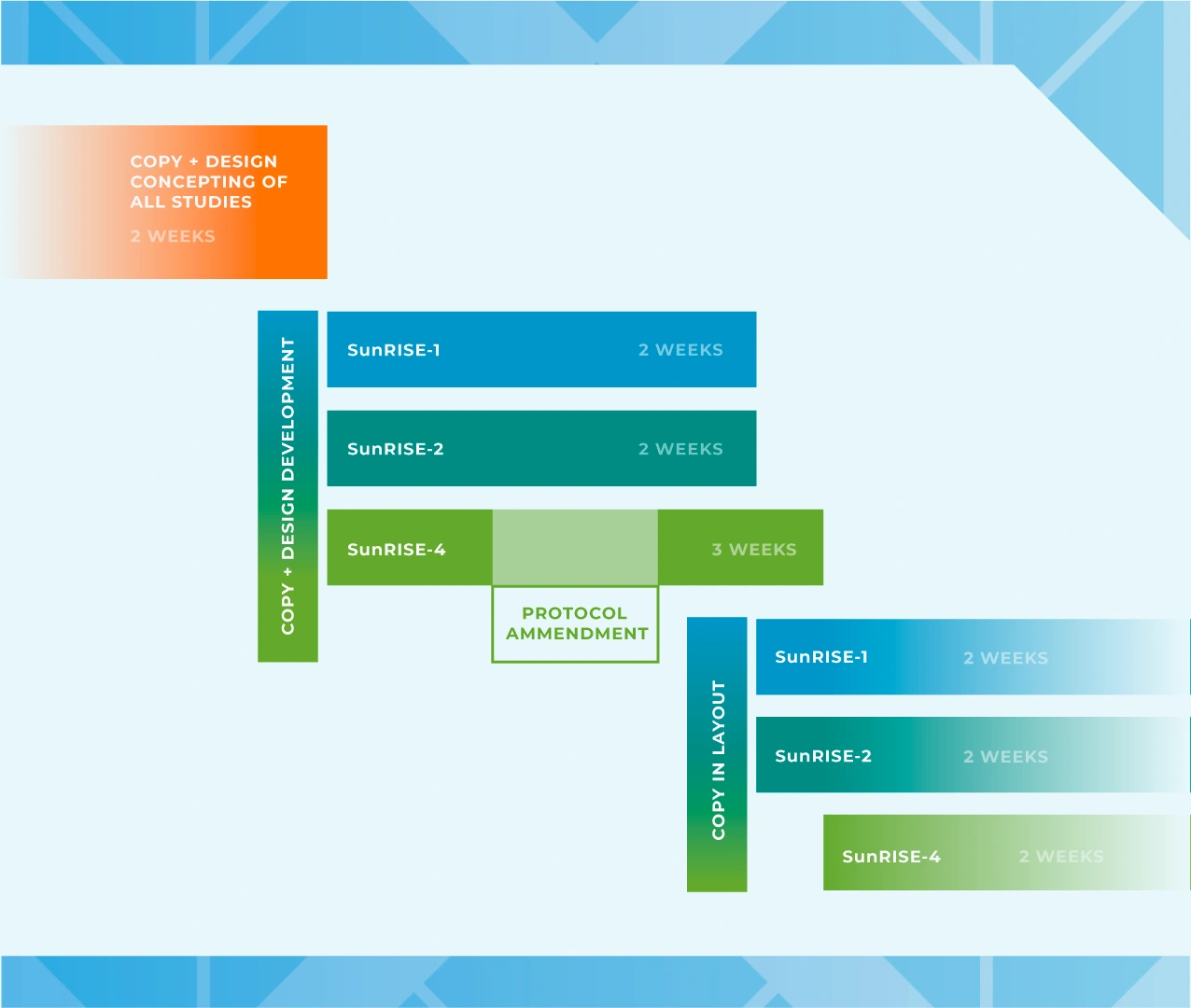
THE PROJECT
Janssen and PRA Health Sciences set out to establish a global brand identity for the suite of SunRISe Studies and enlisted the patient recruitment team at Stark / Raving to deliver. The program encompassed four unique global clinical studies, each with specific criteria for both study participants and clinical teams. Our approach emphasized the significance of inclusivity, striving to represent a diverse patient population and providing accessible information across a range of materials to assist patient in their clinical trial journey.