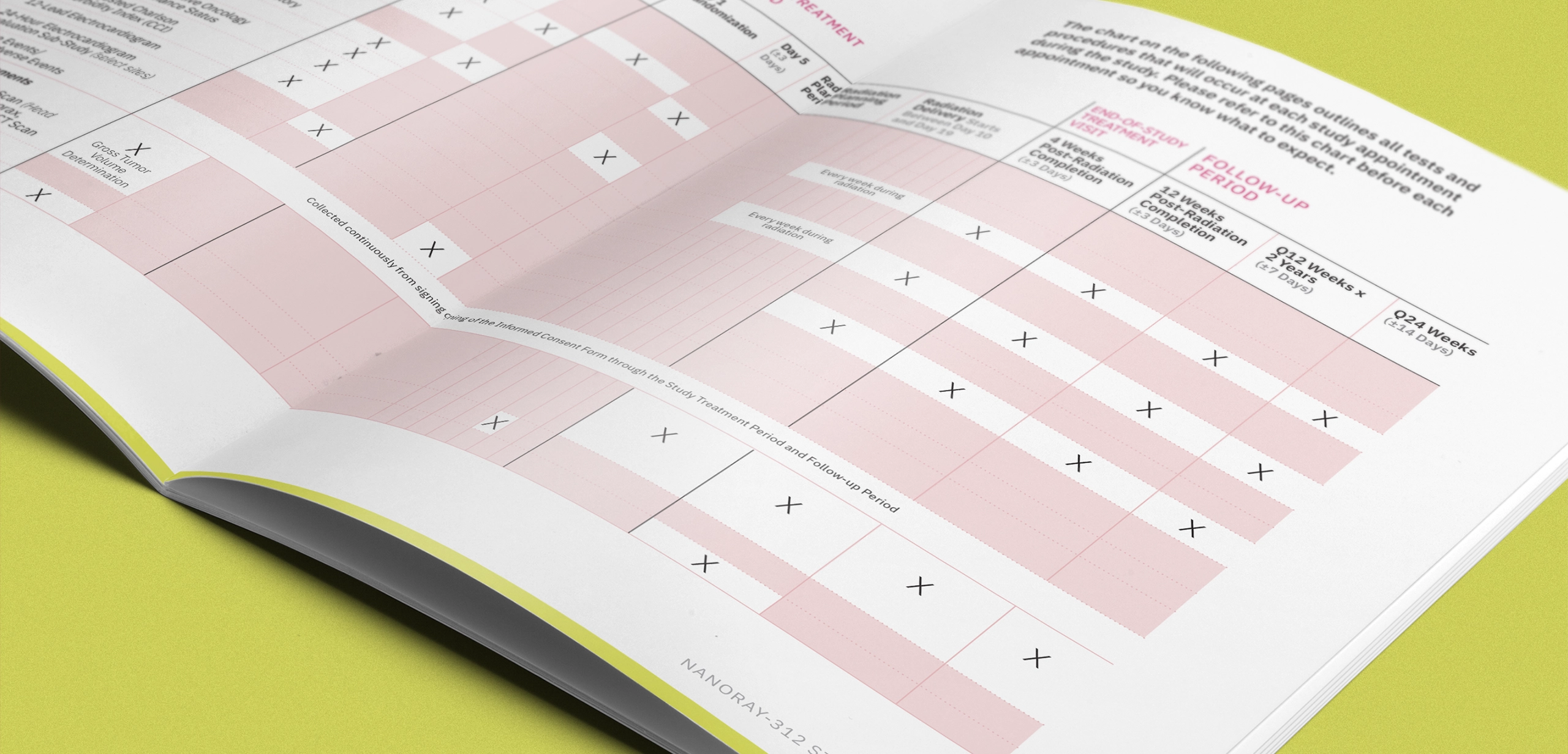
THE CLINICAL TRIAL
Head and neck cancer represents approximately 4% of all cancer cases in the United States, and initial stages of the disease are typically managed through surgery or radiotherapy alone. This clinical trial was examining the effectiveness, performance, and safety of an investigational radioenhancer used in conjunction with radiation therapy and cetuximab, compared to radiation therapy with cetuximab alone. The clinical trial focused on treatment-naïve, elderly participants with locally advanced, platinum-ineligible head and neck squamous cell carcinoma (LA-HNSCC).
THE PROJECT
For over 17 years, Nanobiotix has been at the forefront of nanomedicine, introducing an innovative approach to medicine. Unlike traditional methods rooted in biology or chemistry, their therapeutic technologies leverage physical principles at the nanoscale. Their investigational radioenhancer is being developed for various solid tumor indications and therapeutic combinations. To create a compelling study brand that informs and captivates patients about this promising treatment option, Nanobiotix enlisted the patient recruitment expertise of Stark / Raving. This collaboration aimed to establish a robust study brand that spans their entire clinical trial portfolio, engaging patients and providing them with valuable education on this potential treatment option.